Techlab
A medical device company that designs, develops, and manufactures diagnostic tests for gastrointestinal and infectious diseases.
Employs: 150+
For Tracy Wilkins and David Lyerly, it’s always been all about the science.
The pair met in 1980 at Virginia Tech’s Anaerobe Laboratory, where they were investigating diagnosis and treatments for Clostridium difficile infection. Their partnership resulted in the development of the first commercial diagnostic reagents for C. difficile disease.
Demand for the antiserum grew quickly when hospitals discovered C. difficile was a major cause of hospital-acquired diarrhea and colitis.
Wilkins and Lyerly created TECHLAB Inc. in 1989 with an emphasis on the microbiology of the intestinal tract and improved diagnostics for C. difficile.
“We are totally focused on our relatively untouched niche,” said Wilkins. “Other companies produce a range of products in all sorts of areas, but we are very focused. We are also totally focused on quality, have never had a recall, and have always worked well with the US Food and Drug Administration.”
A place to grow
The company started with a handful of employees, and Lyerly said he and Wilkins “never, ever thought about going anywhere else.”
The company has no difficulty recruiting the talent it needs from Virginia Tech and Radford University.
Lyerly, TECHLAB’s chief scientific officer, said the company benefitted in the early years from contract research and from securing National Institutes for Health-funded grants through the Small Business Innovation Research program.
The company was also able to grow by reaching distribution deals with other companies. TECHLAB’s products are now sold in almost every country in the world.

To ensure the high quality of its products, TECHLAB manufactures most of them. When the company ran out of space, it opened a factory in Radford in 2012, said Rob Day, chief operating officer.
Now more than 150 employees work in Radford and in research and development at the firm’s building in the Virginia Tech Corporate Research Center in Blacksburg. The Radford factory is becoming increasingly automated, which helps the company keep up with demand and the pressures of continually adding new product lines.
The day is rapidly approaching when TECHLAB will need to expand operations in Radford to keep up with the demand of adding new products.
TECHLAB offers its employees state-of-the art facilities that are the envy of larger companies, Day said. “There are no better facilities than what we have set up here in southwest Virginia.”
Rapid Testing
Never, ever thought about going anywhere else.”
—David Lyerly
TECHLAB now produces rapid, non-invasive diagnostics for intestinal inflammation, bacterial infection, and parasitology. The company’s in vitro diagnostics for enteric diseases help determine whether patients have C. diff, E. coli, protozoan parasites, inflammatory bowel disease, or irritable bowel syndrome.
The company has gotten FDA clearance on six tests in the past 14 months, said Dan Delaney, TECHLAB president and CEO.
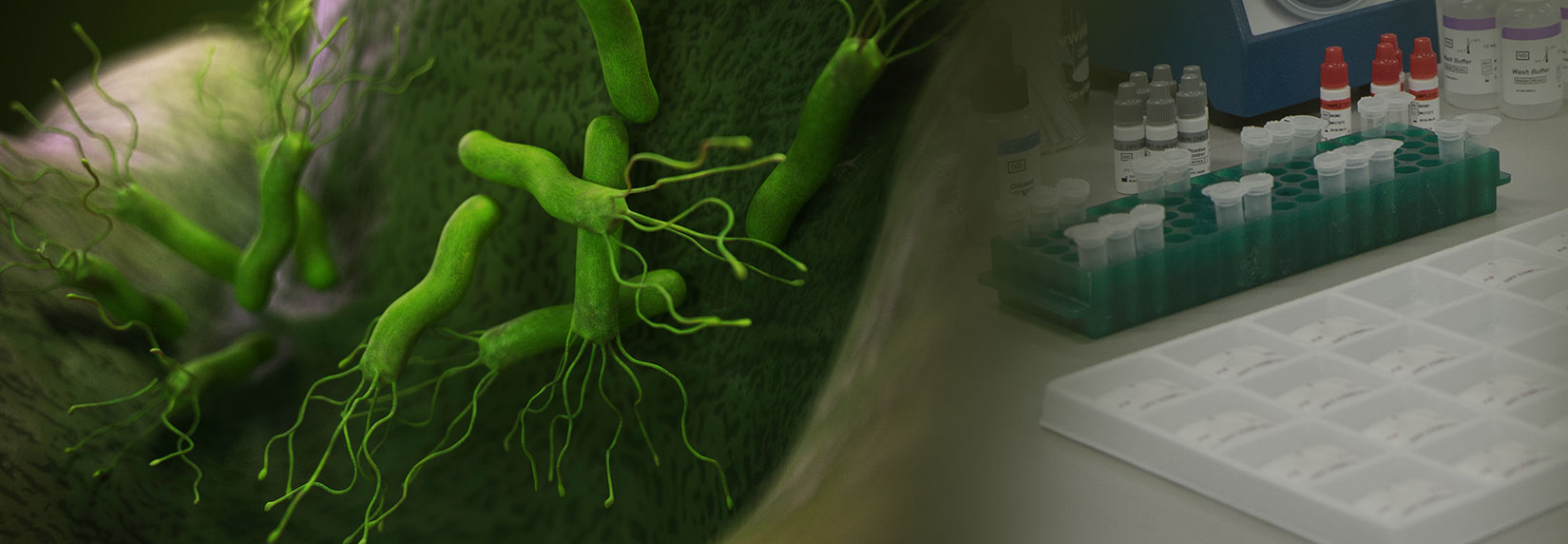
Most recently in August, the company received FDA clearances for its H. PYLORI QUIK CHEK™ and H. PYLORI CHEK™ tests.
Both are designed to help diagnose H. pylori infections, which affect half the world’s population and are responsible for most duodenal and gastric ulcers. The infections are also associated with increased risk for gastric cancer and mucosal associated-lymphoma.

Help for pets
TECHLAB also has created GIARDIA VET CHEK™, which uses antibodies to detect intestinal tract infections in canine and feline fecal samples. The test is the first USDA-licensed test of its kind on the market.
The test identifies Giardia, a parasitic infection that commonly causes diarrhea in dogs and cats.
The company plans to develop more diagnostic tests for veterinary use.
Committed to Virginia
In 2006, Wilkins and Lyerly accepted an investment from Alere Medical, a publicly-traded world leader in diagnostics (now part of Abbott Laboratories). In 2016, Pharos Capital Group acquired a controlling interest in TECHLAB.
Before the Pharos sale, Lyerly and Wilkins walked away from another deal to sell the company when they learned the new owners planned to move the company overseas.
“We came to the conclusion that doing so would defeat everything we ever wanted for TECHLAB, which was to build a biotech leader in Southwest Virginia” said Lyerly. “TECHLAB remains dedicated to the science and focused on doing what it does best.”
Looking ahead
Delaney said TECHLAB, which has an impressive patent portfolio, is building on its successes.
“We are growing beyond GI diseases, creating new tests, and testing new markets,” he said. “We are working to achieve full utilization of the scientific and manufacturing expertise that we have and are expanding our commercial capabilities.”